Abstract
Background
Prothrombin time (PT) is a clot-based assay that remains the clinical standard for diagnosis of coagulopathy. Prolongation of PT can be attributed to decreased fibrinogen (Fg) and dysfibrinogenemia but is commonly interpreted as a deficiency in thrombin (FIIa) generation. Paradoxically, conditions in which PT becomes prolonged (e.g., DIC, trauma) are associated with increased FIIa generation. This suggests that prolonged PT may be misleading, and efforts to improve PT in hypocoagulopathy by promoting FIIa generation may be misguided. We modeled the effects on fibrin polymerization related to varying FIIa, Fg, and Fg degradation products (FDPs, such as D-Dimer) in vitro as a platform for understanding the mechanisms underlying hypocoagulopathy.
Methods
In vitro studies were conducted using prothrombin-deficient plasmas, synthetic plasma (Fg and coagulation factors in HEPES buffered saline), and re-assembled whole blood (plasma with fresh RBCs and platelets). Concentrations of synthetic plasma components were varied to model coagulopathic states (Shaz et al. J Trauma 2011;70(6):1401-7; Johansson et al. Crit Care. 2011;15(6):R272). Assays included thromboelastography (TEG), fibrin polymerization (turbidimetry), FIIa generation (calibrated automated thrombogram, CAT), rheology, and PT/INR (Stago STA-R Evolution).
Results
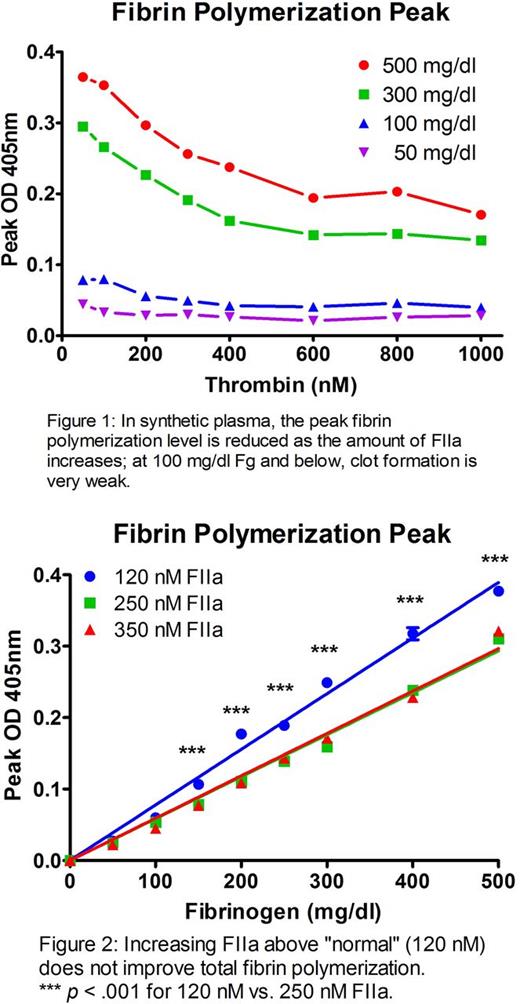
In vitro simulations of coagulopathy illustrated the significance of each factor on coagulation function. A dose response of FIIa (50-1000 nM) in prothrombin-deficient plasma demonstrated that beyond the normal FIIa range (100-200 nM), excess FIIa did not enhance clot characteristics; in fact, TEG maximum amplitude (clot strength) decreased in a FIIa dose-dependent fashion versus the equivalent dose of prothrombin (p < .001).
In synthetic plasma, a similar dose-dependent FIIa effect on fibrin polymerization peak was shown at Fg concentrations of 5 and 3 mg/ml; depleting Fg to 1 mg/ml also showed this effect, but at this level clot formation was extremely poor even with optimal FIIa (Figure 1). The exponential turbidity rise as Fg converts to fibrin resulted in a time-to-peak of ~60 s for all FIIa conc. (time-to-half-peak was < 15 s). For equivalent Fg, increasing FIIa from 120 nM (peak in normal pooled blood) to 250 nM (peak in hemorrhaged coagulopathic patient; Cardenas et al. J Trauma. 2014;77(6):839-45) resulted in decreased total fibrin polymerization (p < .001 between 120 and 250 nM FIIa for all Fg values ≥ 150 mg/dl; Figure 2). With rheology, 150 nM FIIa resulted in rapidly formed clots with a significant decrease in stiffness vs. clots allowed to form by contact activation (135 Pa vs. 4.5 Pa with FIIa; p < .001).
When RBCs and platelets were present, the same FIIa dose-dependent effect existed but was highly mitigated with increasing hematocrit (from 0 to 40%). The inhibitory effect of increased FIIa on fibrin polymerization was enhanced by addition of exogenous FDPs in a model of hyperfibrinolysis.
Conclusions
Prothrombin levels typically drop following severe hemorrhage but are sufficient to produce the active enzyme. These in vitro studies show that the prolongation of PT found in hypocoagulopathy may be a misleading diagnostic; increasing FIIa levels may improve PT but does not improve clot function. The addition of excess FIIa, while rapidly initiating clot formation, results in poorly formed clots.
Excess FIIa generated during injury and hemorrhage combined with production of FDPs is mimicked in these in vitro studies, leading to a hypocoagulable state. Additionally, changes in Fg have profound effects on coagulation within the range of 100-300 mg/dl. The inhibitory effects of excess FIIa are worsened with insufficient Fg, aggravated further by D-dimer. Therefore, increases in PT under these circumstances may be attributed to poor fibrin polymerization.
Hyperfibrinolysis is problematic, not just because of the breakdown of hemostasis-providing clots, but also due to the effects that FDPs have on future clot formation, particularly amplified at low Fg levels.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal